
Defective Medical Device Lawyers
Nationwide Representation for Wrongfully Injured Patients
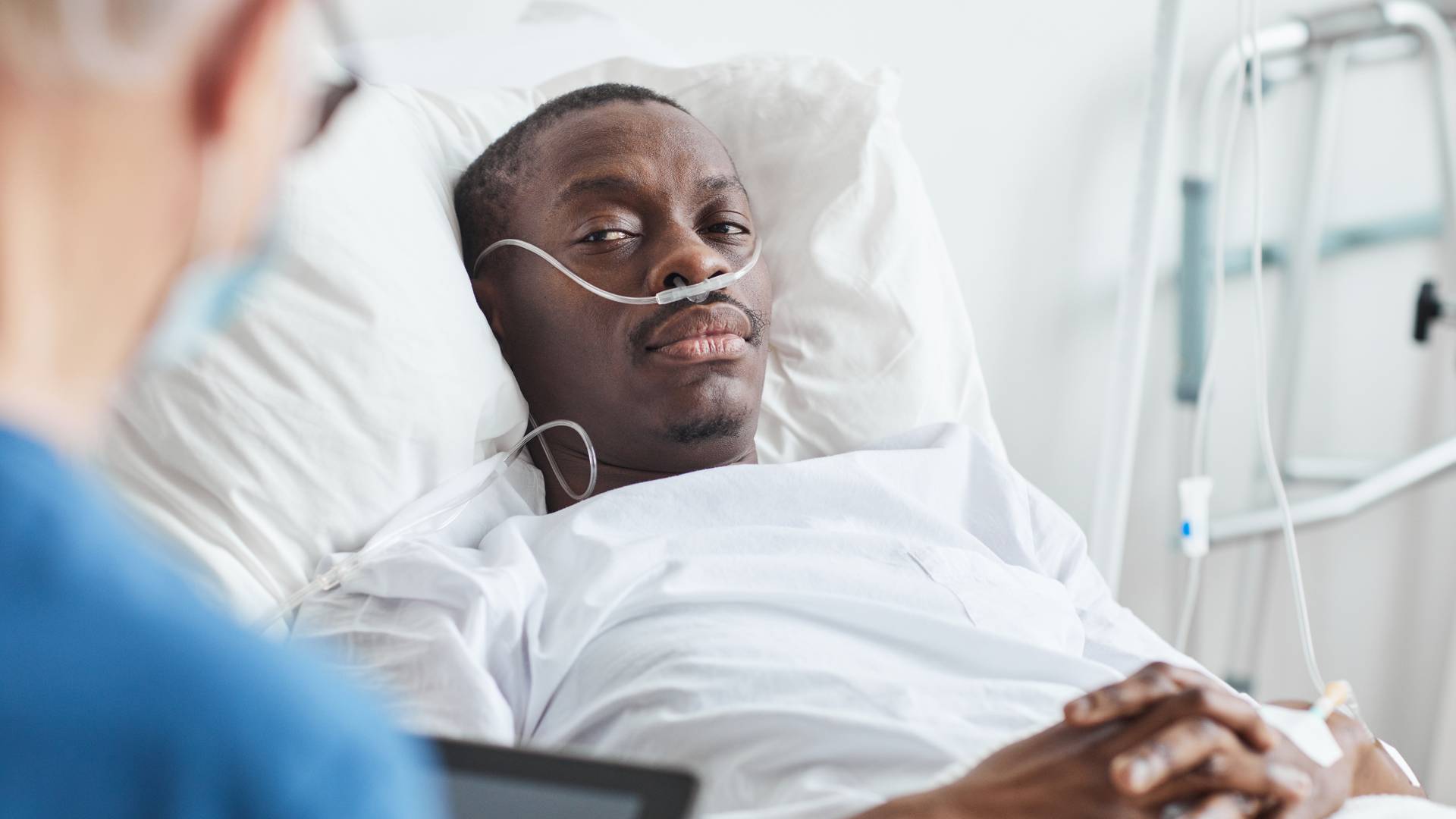
While healthcare innovation can sound new and exciting, the unfortunate reality is that many medical device companies fail to prioritize the well-being of their customers. From marketing a product without adequate testing to being dishonest about risks and side effects, dangerous medical devices can result in significant harm to patients.
At Matthews & Associates, we refuse to let healthcare entities and corporate giants profit at the expense of human lives. Our firm understands the pain and frustration of suffering harm due to dangerous products, which is why our defective medical device attorneys are committed to pursuing justice for patients nationwide. With over 25 years of experience handling mass tort lawsuits, we can aggressively protect your rights while seeking the fair compensation you deserve.
If you were harmed by a dangerous medical device, Matthews & Associates can pursue justice on your behalf. Contact us online to discuss your case.
We're Ready to Fight For You!
-
“Very professional from the beginning”
- Jeffrey E.Very professional from the beginning. always answered questions and had amazing follow through on insuring data submitted was accurate
-
“They are so helpful, kind, and knowledgeable.”
I love these guys! They are so helpful, kind, knowledgeable, smart, and good with people. They answer all your questions and if they don't know the answer, it doesn't take them long to get you the answer! I wouldn't want anyone else to handle my suit. They are AWESOME!- Genie S. -
“Very informative and professional.”
- Lydia C.Very informative and professional. Answer thoroughly all my questions and concerns.
-
“Always available & answers questions promptly.”
I've been working with Matthews & Associates for 2 years. I am impressed that the firm is always available and answers questions promptly. I am very satisfied with the services they provide and would recommend them.- Kweli Y. -
“Matthews & Associates is one of a kind.”
Matthews & Associates is one of a kind. They treat you with respect. They support you when needed. Plus they have locations in Texas and California with wonderful customer service. Bilingual service is always available.- Sorangel T. -
“Matthews and Associates are very informative. Kept me up to date on what was happening with my case.”
- Ron S.Matthews and Associates are very informative. Kept me up to date on what was happening with my case. They were always polite when they were talking to me. Very professional and polite.
What Constitutes a Defective Medical Device?
A medical device is considered defective when it does not perform as intended, resulting in harm or potential harm to the patient.
This can occur due to a variety of reasons, such as:
- Design flaws: A design defect occurs when a medical device poses an unreasonable risk of injury or harm, even if manufactured correctly.
- Manufacturing flaws: A manufacturing defect happens when an error occurs during the device's production process, causing it to deviate from its intended design and harming patients.
- Insufficient labeling: Failing to provide adequate warnings or instructions regarding the device's use can result in marketing defects. This occurs when the manufacturer doesn't properly inform healthcare providers or patients of the potential risks or side effects associated with the device or doesn't provide correct instructions for its use.
As mass tort attorneys and defective device lawyers, our responsibility is to thoroughly investigate these issues to identify defective devices and determine liability. If you suffered a medical device injury, our firm can work tirelessly to maximize the value of your claim by holding all negligent parties accountable.
Take Action – Call Our Team Today!
-
 David P. Matthews Attorney
David P. Matthews Attorney"When the judge read the verdict in Martha Salazar’s case ($73 Million), and we learned the jury ordered [the medical device maker] to pay Martha, (we) knew they believed every word she said. We realized they knew the defendant was truly guilty."
Assistant Editor

Who Is Liable for Dangerous Medical Devices?
Depending on the circumstances, various parties can be liable for defective medical devices.
Common liable parties in defective device lawsuits include:
- Device manufacturers: In many cases, the company that produces and sells the medical device can be liable for defects.
- Medical institutions: Hospitals, clinics, and other healthcare organizations have an obligation to ensure the equipment they use is safe. If they fail to uphold this duty, such as knowingly using a dangerous device during treatment, these institutions can be liable for defective devices.
- Doctors and surgeons: Medical practitioners can be liable in dangerous device lawsuits if they fail to inform patients about the potential risks of a medical device or improperly install the device, causing harm to the patient.
- Testing laboratories: Labs involved in the safety and efficacy testing of dangerous medical devices can be held accountable if they provide misleading or incorrect results, leading to the use of unsafe devices.
- Sales representatives: Representatives who fail to disclose all necessary information or give misleading information about a product can be liable for damages.
- Component suppliers: Companies supplying components or materials used in the manufacturing of a defective device can be liable if their component leads to the device's defect.
Over $10 Million Recovered for Injured Patients
At Matthews & Associates, we know that no amount of compensation can fully make up for the tremendous pain and suffering patients endure due to dangerous medical devices. If you were harmed by a medical device that was falsely promoted as safe and effective, we share your anger—and we’re here to help you fight back.
Since 2006, our fearless lawyers have sought compensation for patients harmed by negligence in California, Texas, New York, and beyond. With hundreds of thousands of cases handled, clients trust us to prioritize their best interests when companies fail to consider their well-being. If you suffered a medical device injury, turn to a firm with national experience in mass tort lawsuits to demand justice.
Our experienced attorneys represent patients across the country in dangerous medical device lawsuits. Call (888) 923-7001 to schedule a free consultation.

Offices in California & Texas
PROVIDING LEGAL REPRESENTATION AND GUIDANCE NATIONWIDE
-
Firm Roots
David P. Matthews founded our firm after 16 years as a partner at a prominent Houston law firm.
-
ExperienceWe have longstanding experience of representing injured people in serious auto and truck accident claims, workplace injuries, aviation accident claims, dialysis injury claims, and a broad array of medical device claims, from transvaginal mesh to hip replacement surgery injury claims.
-
Nationwide RepresentationIn dangerous pharmaceutical drug cases, medical device cases, and several others including FELA lawsuits, and many more, we also represent clients on a nationwide basis.








.2401021128579.png)
.2401021129535.png)
.2401021130548.png)

