
Dangerous Drug Attorneys
Trial Lawyers Representing Victims of Dangerous Drugs Nationwide
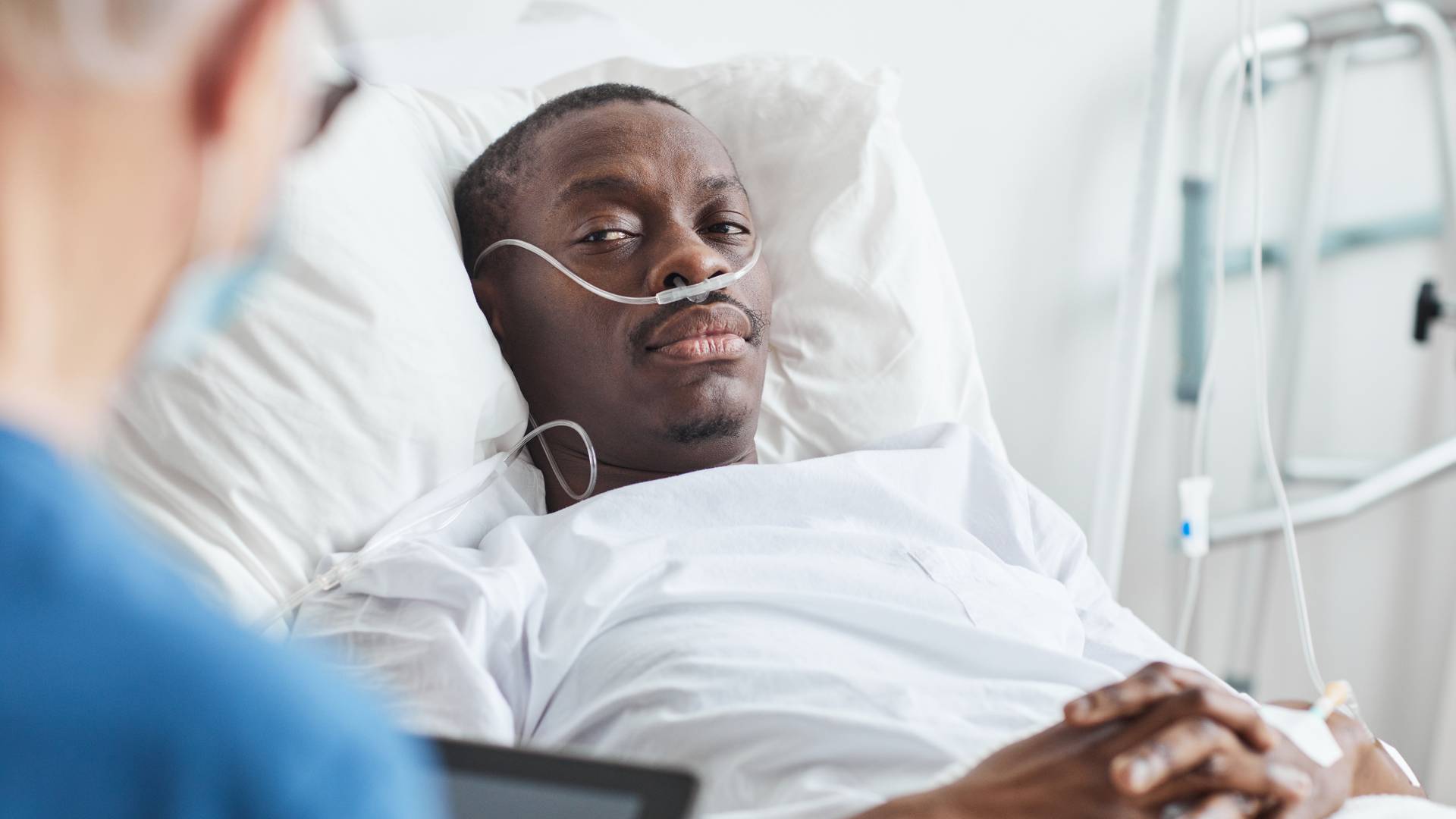
Medications often serve as remedies for our health issues, acting as healing tools to improve our lives. However, what if these so-called "solutions" worsen the problem or give rise to new health issues?
Regrettably, this is the reality faced by victims of hazardous drugs. These can range from common over-the-counter medications like Tylenol to elective procedures involving substances like Botox, as well as prescription drugs such as Abilify.
When individuals suffer harm as a result of medications like these, they deserve fair and equitable compensation. This is where our dedicated team at Matthews & Associates comes into play. We offer legal guidance and representation to those who have been injured due to the usage of hazardous drugs.
With Matthews & Associates on your side, you'll find a team of dangerous drug lawyers committed to helping you navigate the complexities of the legal landscape surrounding your case.
To schedule a free consultationwith one of our dangerous drug attorneys, call (888) 923-7001today.
We're Ready to Fight For You!
-
“Always available & answers questions promptly.”
I've been working with Matthews & Associates for 2 years. I am impressed that the firm is always available and answers questions promptly. I am very satisfied with the services they provide and would recommend them.- Kweli Y. -
“Very professional from the beginning”
- Jeffrey E.Very professional from the beginning. always answered questions and had amazing follow through on insuring data submitted was accurate
-
“Very informative and professional.”
- Lydia C.Very informative and professional. Answer thoroughly all my questions and concerns.
-
“Matthews & Associates is one of a kind.”
Matthews & Associates is one of a kind. They treat you with respect. They support you when needed. Plus they have locations in Texas and California with wonderful customer service. Bilingual service is always available.- Sorangel T. -
“They are so helpful, kind, and knowledgeable.”
I love these guys! They are so helpful, kind, knowledgeable, smart, and good with people. They answer all your questions and if they don't know the answer, it doesn't take them long to get you the answer! I wouldn't want anyone else to handle my suit. They are AWESOME!- Genie S. -
“Matthews and Associates are very informative. Kept me up to date on what was happening with my case.”
- Ron S.Matthews and Associates are very informative. Kept me up to date on what was happening with my case. They were always polite when they were talking to me. Very professional and polite.
Representing Clients in a Range of Dangerous Drug Cases
At Matthews & Associates, we represent clients across a broad range of dangerous drug cases.
We have successfully assisted clients in pursuing compensation for injuries associated with the following medications:
- Abilify
- Accutane
- Aciphex
- Actemra
- Acthar
- Actos
- Belviq Diet Drug
- Benicar
- Botox
- Depo-Provera
- Elmiron
- Farxiga
- Fosamax
- GranuFlo/Dialysis Injury
- Lipitor
- Mirena IUD
- Nexium
- Nuplazid
- NuvaRing
- Onglyza
- Potiga
- Pradaxa
- Prevacid
- Prilosec
- Protonix
- Raptiva
- Reglan
- Risperdal
- Steglatro
- Suboxone
- The Shingles Vaccine Zostovax
- Taxotere Hair Loss
- Testosterone Supplement (Low-T Injury)
- Truvada HIV Drug
- Tylenol – Acetaminophen
- Xarelto
- Xeljanz
- Yaz
- Zantac
- Z-Pak
- Zostovax Shingles Vaccine
If you have been injured by a drug not listed here, we encourage you to reach out for a free consultation. Our team is prepared to evaluate your situation and help you determine the best course of action moving forward.
Take Action – Call Our Team Today!
-
 David P. Matthews Attorney
David P. Matthews Attorney"When the judge read the verdict in Martha Salazar’s case ($73 Million), and we learned the jury ordered [the medical device maker] to pay Martha, (we) knew they believed every word she said. We realized they knew the defendant was truly guilty."
Assistant Editor

When is a Drug Considered Dangerous?
Legally speaking, a drug could be labeled as dangerous if it's linked to severe medical complications despite being used as directed. These complications can range from physical ailments, such as organ damage or heart conditions, to mental health issues, including depression or suicidal thoughts. It's crucial to remember that a drug's dangerous nature often depends on individual health circumstances and the specific way the drug interacts with one's body, which can vary widely among users.
Significantly, even drugs that have been approved by the Food and Drug Administration (FDA) can later be categorized as dangerous. This can occur when post-market surveillance reveals new risks or when the rate of complications is higher than previously reported during clinical trials. In such cases, the FDA may issue safety warnings, mandate additional label warnings, or even recall the drug. It's in these scenarios that legal intervention becomes essential to protect the rights of those affected and to seek appropriate redress for their injuries.
Pursuing Justice & Fair Compensation in Dangerous Drug Cases across the United States
You don't have to face the journey towards justice and rightful compensation alone. Allow the experienced dangerous drug attorneys at Matthews & Associates to serve as your guiding light in your pursuit of justice. With a deep passion and unwavering commitment, we stand by your side, dedicated to your cause.
To schedule a free consultation, call (888) 923-7001 or contact us online today.

Offices in California & Texas
PROVIDING LEGAL REPRESENTATION AND GUIDANCE NATIONWIDE
-
Firm Roots
David P. Matthews founded our firm after 16 years as a partner at a prominent Houston law firm.
-
ExperienceWe have longstanding experience of representing injured people in serious auto and truck accident claims, workplace injuries, aviation accident claims, dialysis injury claims, and a broad array of medical device claims, from transvaginal mesh to hip replacement surgery injury claims.
-
Nationwide RepresentationIn dangerous pharmaceutical drug cases, medical device cases, and several others including FELA lawsuits, and many more, we also represent clients on a nationwide basis.








.2401021128579.png)
.2401021129535.png)
.2401021130548.png)

