(January 31, 2020) – A California judge fined Johnson & Johnson subsidiary Ethicon $344 million yesterday for misleading consumers about the actual risks of the company’s pelvic mesh products. San Diego Superior Court Judge Eddie Sturgeon presided over the two-month bench trial in the first case of its kind brought by a state’s attorney general.
Judge Sturgeon sided with California Attorney General Xavier Becerra. In his 88-page statement of decision, the judge ruled J&J and Ethicon violated the state’s laws against unfair competition and false advertising.
Judge Sturgeon wrote that the state of California had “proven by a preponderance of the evidence that defendants deceptively marketed their pelvic mesh products in the state (and) that their marketing was likely to deceive reasonable doctors and reasonable lay consumers, including potential patients and their friends and family, about the risks and dangers of these products.”
TVT and Prolift Meshes for SUI and POP
The judge found the defendants had lied to California consumers and doctors about the safety of two J&J / Ethicon lines of mesh products made with polypropylene (plastic): 1) Tension-free Vaginal Tape (TVT), launched in 1998 to treat stress urinary incontinence (SUI); and 2) Prolift, launched in 2005 to treat pelvic organ prolapse (POP). J&J cased selling the latter in 2012.
Mr. Becerra said in a post-decision statement that J&J knew of the dangers its mesh products posed, but nevertheless placed company profits over women’s health.
J&J Intentionally Concealed Dangers of Mesh Products
The California attorney general said: “Johnson & Johnson intentionally concealed the risks of its pelvic mesh implant devices. It robbed women and their doctors of their ability to make informed decisions about whether to permanently implant the products in patients’ bodies. Today we achieved justice for the women and families forever scarred by Johnson & Johnson’s dishonesty.”
Ethicon plans Appeal
Ethicon said  in a statement yesterday that it plans to appeal the decision. The company maintained — as its lawyers had throughout the trial — that the state hadn’t shown evidence proving California doctors or patients were actually deceived by J&J’s mesh marketing.
in a statement yesterday that it plans to appeal the decision. The company maintained — as its lawyers had throughout the trial — that the state hadn’t shown evidence proving California doctors or patients were actually deceived by J&J’s mesh marketing.
A company spokesperson said: “Ethicon responsibly communicated the risks and benefits of its transvaginal mesh products to doctors and patients, and the decision disregards the company’s full compliance with U.S. Food and Drug Administration laws on medical device communications and the appropriateness of its actions.”
J&J/Ethicon said testimony from California doctors showed they used the products successfully. The company disagreed with the state’s allegations, and noted the state lacked California witnesses with first-hand experience using the devices or seeing Ethicon’s marketing.
J&J Doctor called as Hostile Witness
In his statement of decision, however, Judge Sturgeon wrote that he was persuaded by the state’s evidence. He repeatedly referenced testimony from Dr. Piet Hinoul, a former gynecologist and Ethicon’s current global head of preclinical, clinical and medical affairs.
The state called Dr. Hinoul to the stand as a hostile witness. He testified that J&J knew from the time it launched the TVT in 1998 that its mesh slings caused severe, long-term complications such as the shrinking of the tissue surrounding the mesh, chronic pain, and pain during sex.
Judge Sturgeon: J&J Risks “deceptively incomplete”
Judge Sturgeon wrote that J&J kept its knowledge of the most serious risks secret from doctors and patients. He said the company sent “deceptively incomplete” information in its Instructions For Use that accompanied each Ethicon device. The IFU is meant to help physicians understand risks.
The judge wrote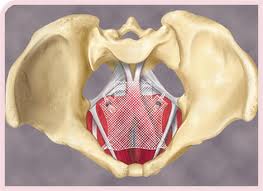 that trial evidence showed, “rather than disclose what it knew about some of the severe risks of pelvic mesh in its labeling and marketing materials, J&J has instead taken active, willful measures for nearly twenty years to suppress information and conceal serious risk and complication information from physicians and patients.”
that trial evidence showed, “rather than disclose what it knew about some of the severe risks of pelvic mesh in its labeling and marketing materials, J&J has instead taken active, willful measures for nearly twenty years to suppress information and conceal serious risk and complication information from physicians and patients.”
J&J continues to Sell TVT Mesh
The state also requested that Judge Sturgeon issue an injunction to bar J&J from making deceptive statements about its mesh. In answer to that request, Judge Sturgeon wrote that because J&J/Ethicon is still selling the TVT product, and has not acknowledged or disavowed its deceptive marketing, an injunction might be warranted.
Judge Sturgeon asked the parties to file supplemental briefs on the issue of injunctive relief by Feb. 18.
The state had sought nearly $800 million in civil penalties when the case was first brought by former California Attorney General Kamala Harris in 2016.
The case is California v. Johnson & Johnson et al., case number 37-2016-00017229-CU-MC-CTL, in the Superior Court of the State of California, County of San Diego.
Johnson & Johnson has also been sued by several thousand women across the country who allege that they’ve suffered injuries as a result of being implanted with the company’s TVT and other transvaginal mesh products.
RELATED
- Transvaginal Mesh Lawsuit |Pelvic Mesh Lawsuit
- States sue J&J Transvaginal Mesh Maker
- Johnson & Johnson, Ethicon Mesh Losses Mount
- Jury awards $73 Million Verdict in Pelvic Mesh Case
- Mesh Verdict stands against Johnson & Johnson
- &J fined $344M by Calif. Judge for False Pelvic Mesh Marketing

by Matthews & Associates




