 (Feb 6. 2019) The FDA issued a letter today which warns that breast implants may increase the risk of Associated-Anaplastic Large Cell Lymphoma. The agency sent the letter to health care providers alerting them to the problem and the potential risks to their patients of breast implants.
(Feb 6. 2019) The FDA issued a letter today which warns that breast implants may increase the risk of Associated-Anaplastic Large Cell Lymphoma. The agency sent the letter to health care providers alerting them to the problem and the potential risks to their patients of breast implants.
All Breast Implants Included
The letter stated that the FDA “wants to increase awareness about an association between all breast implants [emphasis ours], regardless of filling or texture, and Breast Implant Associated-Anaplastic Large Cell Lymphoma (BIA-ALCL).”
The agency said it had received reports indicating that patients with breast implants face an increased risk of developing this disease within the scar capsule adjacent to the implant.
The FDA wrote: “We want all healthcare providers to be aware of BIA-ALCL, particularly in patients with new swelling, lumps, or pain around breast implants, to expedite diagnosis of this malignancy. We are also asking health care providers to report to the FDA cases of BIA-ALCL in patients with breast implants. This includes reporting individual cases as well as rates you may have experienced during your practice.”
Associated-Anaplastic Large Cell Lymphoma
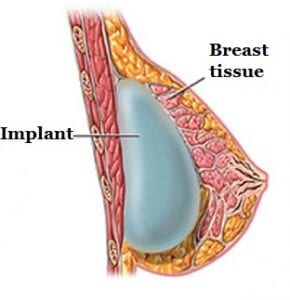
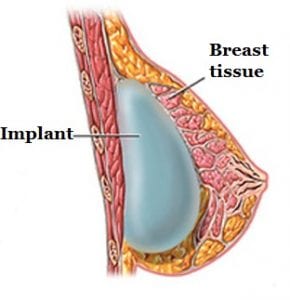
BIA-ALCL is not a cancer of the breast tissue but a type of lymphoma. Breast implants are inserted behind the breast tissue or under the chest muscle (see photo). A fibrous scar called a capsule then develops around the implant, separating it from the rest of the breast. Cases of BIA-ALCL have typically been found adjacent to the implant and contained within that fibrous capsule.
The FDA says a significant amount of medical literature has been published since its 2011 report on BIA-ALCL. It includes more case histories and comprehensive reviews of the natural history and long-term outcomes of BIA-ALCL. Current literature reports various estimates for the incidence of BIA-ALCL. These estimated incidence rates range from 1 in 3,817 patients to 1 in 30,000 (Clemens et al, 2017; Loch-Wilkinson et al, 2017; De Boer et al, 2018).
Textured vs. non-Textured Implants
Most patients who develop BIA-ALCL have received textured implants, but BIA-ALCL reports have also involved patients with smooth-surfaced implants, and many diagnostic reports do not detail the implant’s surface texture.
660 MDRs for BIA-ALCL, 9 Deaths
The FDA said it has received a total of 660 Medical Device Reports (MDRs) of BIA-ALCL. The agency has reviewed the “660 MDRs to remove duplicate reports and to control for MDRs in which a BIA-ALCL diagnosis was confirmed by: a physician, positive pathology/cytology test results, or positive for biomarker CD30 and negative for biomarker ALK. The FDA’s additional data analysis identified 457 unique MDRs for BIA-ALCL, including the death of nine patients which may be attributable to BIA-ALCL. However, it is important to note that at the time of diagnosis, patients may have their original breast implants or they may have had one or more replacements.”
Risk Factors for BIA-ALCL
Recent journal articles explore possible risk factors for developing BIA-ALCL, including the methods used to create the textured surface of the implant and the possible role of biofilm.
Treatment for BIA-ALCL
Most of the published information regarding treatment involves removal of the implant and the capsule surrounding the implant, and in some patients, treatment with chemotherapy and radiation.
1.5 Million Breast Implants Yearly – Increased Risks
Though FDA says the number of identified cases of BIA-ALCL is small compared to the estimated 1.5 million patients who receive breast implants worldwide each year, confirmed data and published information that the agency has reviewed suggests patients with breast implants have an increased risk of BIA-ALCL.
Related
- FDA : Breast Implant Complications

by Matthews & Associates