 Blood pumps can kill patients in their homes when they try to change the controller. At least 26 advanced heart failure patients have died, and 19 have been injured, trying to change the controller on Abbott Laboratories’ HeartMate II blood pumps.
Blood pumps can kill patients in their homes when they try to change the controller. At least 26 advanced heart failure patients have died, and 19 have been injured, trying to change the controller on Abbott Laboratories’ HeartMate II blood pumps.70 Reports of Malfunction
After 70 reports of malfunctions, Abbott released new software and alarm guides concerning nearly 29,000 devices to doctors on March 30, 2017. The malfunctions were reported following unsuccessful controller replacement.
HeartMate II Left Ventricular Assist Device
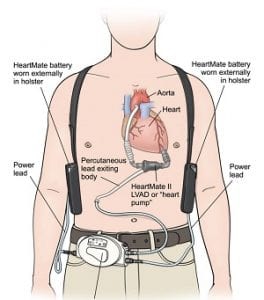
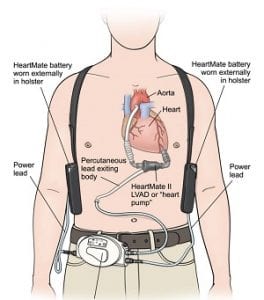
The HeartMate II Left Ventricular Assist Device (AVLAD) blood pump is an implantable medical device that Abbott acquired from St Jude Medical in January 2017. Implanted inside a patient, it works to pump blood around the body when a heart is too weak to work alone. Users must replace an external Pocket System Controller needed to operate the device. Abbott has advised that replacements be conducted only by clinical professionals.
Because of user dangers, the FDA in March 2017 issued a Class 1 recall – its most serious – which urged users not to change controllers themselves.
The recall notice said: “Patients may sometimes need to change to their backup system controller during the course of ventricular assist therapy. (The) change should be done quickly and in the hospital, because it can present a significant challenge to patients that are elderly and/or untrained. For these patients, a slow or improper…changeover places them at risk of serious injury or death.”
The expensive HeartMate II device was designed to keep people alive until they could receive a heart transplant; however, the FDA approved its use as a “destination therapy” in those who do not qualify for such a transplant.
Abbott’s Response
Abbott responded to the FDA’s decision by noting that it is currently engaged in continuing efforts to ensure the device’s safety. Abbott spokesman Justin Paquette said: “We are updating its alert guides, conducting a software upgrade and adding controller alignment markings for the HeartMate II System Controller, as part of a continued effort to ensure patients are successfully able to exchange their pocket controller in emergency situations. Despite past efforts to improve training and education, we are aware of patients experiencing a very low level of adverse events as a result of unnecessary patient controller exchanges.”
Mr. Paquette added that this Class I recall was not about recalling products, but “communication to physicians so they can ensure their patients have their controller exchange completed in a clinical setting In the event that patients require an immediate controller exchange, Mr. Paquette advised, “We have also updated our software and controller alerts to help guide patients to talk to their physician when the time is approaching to have their controller exchanged.”
Medical Device Injury Lawyers
Matthews & Associates Law Firm, a longtime leader in medical device litigation, is investigating Heartmate II Blood Pump problems. Contact the law firm for a free legal consultation.
Related
- Medical Device Lawyer

by Matthews & Associates