 (April 18, 2018) The first Cook IVC filter lawsuit to be tried in state court in the U.S. will begin on May 7, 2017 in Houston, Texas. The plaintiff is a Texas firefighter who has brought suit against Cook Medical Inc., based in Bloomington, Indiana. He is represented by David Matthews of Matthews & Associates Law Firm, in Houston, as well as Tim Goss of Freese and Goss in Dallas, Texas. The trial is expected to last two weeks.
(April 18, 2018) The first Cook IVC filter lawsuit to be tried in state court in the U.S. will begin on May 7, 2017 in Houston, Texas. The plaintiff is a Texas firefighter who has brought suit against Cook Medical Inc., based in Bloomington, Indiana. He is represented by David Matthews of Matthews & Associates Law Firm, in Houston, as well as Tim Goss of Freese and Goss in Dallas, Texas. The trial is expected to last two weeks.
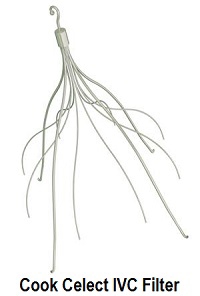
The lawsuit petition states that the plaintiff was surgically implanted with a Cook Celect inferior vena cava filter at Methodist Hospital. On or about April 23, 2015, surgeons attempted to remove the Celect IVC filter but were “unsuccessful because the filter had tilted and become embedded in the wall of Plaintiff’s inferior vena cava.”
The vena cava is the blood vessel located beneath the heart which returns blood to the heart from the lower body.
Relevant facts regarding IVC filter removal as stated in the petition show the difficulty of removing the filter from the plaintiff.
Attempts to remove Plaintiff’s Celect IVC Filter
Approximately two months after the Celect filter was implanted, an interventional radiologist attempted to remove it. He was unable to remove the filter because it had tilted and the tip was embedded into the wall of Plaintiff’s vena cava. After multiple unsuccessful attempts to retrieve the filter via access through Plaintiff’s jugular vein, he aborted the procedure.
In June 2015, another jugular removal procedure was attempted by an interventional radiologist. In connection with this attempted removal, imaging revealed that at least two of the filter legs had perforated through the wall of Plaintiff’s vena cava. This retrieval attempt was also unsuccessful.
Finally, on July 20, 2017, Plaintiff had a life-threatening, invasive open surgery to remove the perforated Cook IVC filter. During the open removal surgery, it was determined that Plaintiff had at least three IVC filter legs poking outside of his vena cava: one in his duodenum (bowel); one beginning to perforate his aorta; and one inside his lumbar vein, which had to be resected for the filter leg to be removed. The degree to which Plaintiff will recover from surgery, and the lingering effects of the perforated filter struts, has yet to be determined.
The petition further alleges that the plaintiff is now at risk for future migrations, perforations, and/or fractures from the retained filter. He also faces numerous other health risks, including increased risk of clots and risk of death. He will “require ongoing medical care and monitoring for the rest of his life and may ultimately require additional surgery in an attempt to remove the filter.”
The lawsuit petition alleges that the implanting doctor knew or should have known, inter alia, several things, including that:
- only certain patients were appropriate candidates for an IVC filter.
- the longer a Celect IVC filter remains in the body, the higher the risk of device fracture or failure.
- the Celect filter is not effective to prevent clots and/or increases the risks of clots.
- safer alternatives to the Celect IVC filter existed.
- IVC filters, such as the Celect filter, should not be used in certain patients, such as the plaintiff.
Cook Celect IVC Filter Problems
The petition alleges that Cook Celect filters frequently tilt, migrate, perforate or fracture and thus, involve a high and increasing degree of risk to a patient who has been implanted with a Celect filter.
The petition further claims that Cook knew or should have known that the Celect device was defective and unreasonably dangerous for several reasons, inter alia, including:
• Cook failed to conduct any clinical testing, such as animal studies, to see how the device would function once permanently implanted in the human body;
• The Celect filter had a high rate of fracture, migration, and excessive tilting and perforation of the vena cava wall once implanted. Such failures exposed patients to serious injuries, including: death, hemorrhage; cardiac/pericardial tamponade; cardiac arrhythmia and other symptoms similar to myocardial infarction; severe and persistent pain; perforations of tissue, vessels, and organs; inability to remove the device;
• Cook knew or should have known that certain conditions or post-implant procedures such as morbid obesity or open abdominal procedures could affect the safety and integrity of the device;
• Cook knew or should have known these risks for the Celect filter were and are substantially higher than other similar devices;
• Cook knew or should have known the Celect filter contained conditions which resulted in the device not performing as safely as the ordinary customer would expect;
• Despite being aware of these risks, Cook misrepresented, omitted, and/or failed to provide adequate warnings of these risks or instructions for safe use;
• Cook failed to issue a recall of the Celect filter or otherwise notify consumers that a safer device was available.
IVC Filters
IVC filters first hit the medical market in the late 1960s. Several different medical device makers, including C.R. Bard, have introduced several different designs.
IVC filters are allegedly designed to filter or “catch” blood clots (called “Thrombi”) that travel from the lower portions of the body to the heart and lungs. The filters are designed to be implanted either permanently or temporarily within the vena cava.
The inferior vena cava is a vein that returns blood to the heart from the lower portions of the body. In certain people, for various reasons, thrombi travel from the vessels in the legs and pelvis, through the vena cava and into the lungs. These thrombi often develop in the deep leg veins. They are called “deep vein thrombosis” or “DVT.” Once they reach the lungs they are called “pulmonary emboli.” Pulmonary emboli present grave risks to human health, including the risk of death.
Someone who undergoes knee or hip joint replacement is at risk for developing DVT/PE. Obese patients are also at risk. So too are those who have vascular diseases, or those who have experienced previous strokes. Several other conditions also predispose some people to DVT/PE.
At-risk people may be prescribed medications like Heparin, Warfarin, or Lovenox to regulate the blood clotting factors. Some at high risk who cannot manage with medications may receive an IVC filter in an effort to prevent thromboembolic events.
Wide Use with no Evidence of Efficacy
IVC filters have been implanted by the hundreds of thousands despite there being no reliable evidence which proves their safety and effectiveness. No evidence proves that IVC filters are worth their risks.
Evidence of harm without evidence of benefit
A 2013 paper published in the Journal of the American Medical Association by Vinay Prasad, MD and others offers a stern critique of IVC filters. The authors declare, “While the benefits of the IVC filter are hard to assess, the complications are evident.” They conclude their paper with a warning: “Follow current standard of care and place filters where guidelines advise, or do not place filters, after informed consent informs patients that there is evidence of harm without evidence of benefit.”
First Cook IVC Filter State Trial
The Houston trial is expected to last two weeks.
Related
- IVC Filter Lawsuit| Lawyer
- Cook IVC Filter Attorney
- Retrievable Filters not FDA approved
- IVC Filter Controversy Rages
- No Evidence Blood Clot Filters help

by Matthews & Associates