Johnson & Johnson also continues Litigation
As the total damages assessed against transvaginal mesh manufacturers in just six mesh trials so far approaches $100 Million, several manufacturers have opted to settle thousands of injury cases with women who have filed them. At the same time, some transvaginal mesh makers – such as Boston Scientific and Johnson & Johnson – have vowed to fight on one case at a time. At least six mesh trials are scheduled for the next few months in the United States against Johnson & Johnson and Boston Scientific, Corp. Boston Scientific will defend its plastic mesh in court again next month in Dallas over claims that its plastic mesh product is defective and dangerous. The next transvaginal mesh trial is set to begin in less than a month. The next Johnson & Johnson Ethicon trial will begin Nov. 2014.
Transvaginal Mesh Trials Schedule
Figueroa vs. Boston Scientific, Corp. – 10/8/14
 Fugueroa vs. Boston Scientific is scheduled to start Oct. 10, 2014 in Judge Ken Molberg’s 95th Judicial District Court in Dallas County, just a block from the infamous Dealey Plaza (where JFK was slaughtered by crossfire in 1963). A Boston Scientific plastic mesh sling was implanted in Ms. Figueroa in June 2011 when she was 34 years old. The Solyx SIS – Single Incision Sling – was meant to treat her stress urinary incontinence. When the sling failed to work as advertised and caused her pain, she had corrective surgery in an attempt to remove it in Sept. 2011. She needs at least one more surgery to remove additional portions of the mesh and the permanent anchoring heads in her obturator foramen and obturator muscle.
Fugueroa vs. Boston Scientific is scheduled to start Oct. 10, 2014 in Judge Ken Molberg’s 95th Judicial District Court in Dallas County, just a block from the infamous Dealey Plaza (where JFK was slaughtered by crossfire in 1963). A Boston Scientific plastic mesh sling was implanted in Ms. Figueroa in June 2011 when she was 34 years old. The Solyx SIS – Single Incision Sling – was meant to treat her stress urinary incontinence. When the sling failed to work as advertised and caused her pain, she had corrective surgery in an attempt to remove it in Sept. 2011. She needs at least one more surgery to remove additional portions of the mesh and the permanent anchoring heads in her obturator foramen and obturator muscle.
The Solyx sling is made by Boston Scientific, the same company which was ordered on Sept. 8, 2014 to pay a Texas woman $73.4 Million for what a 12-person jury determined to be a defective product.
Boston Scientic’s web site touts Solyx as a “Single incision sling system designed to offer a procedure with fewer steps. The mesh carrier tip snap-fits to the delivery device allowing for advanced control with micro-adjustability during placement.” Ms. Figueroa’s attorneys are expected to question the safety and effectiveness of the Solyx product, as well as the adequacy of its attendant warnings to prospective customers.
Ms. Figueroa is represented by the same trial firms that won the $73 Million verdict in Dallas in Sept. – Matthews & Associates and Freese & Goss.
Ramirez vs. Johnson & Johnson – 11/3/14
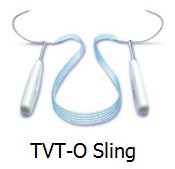 Ramirez vs. Johnson & Johnson (also Reyes, et al.) is scheduled to begin Jan. 12, 2015 in San Antonio, in 438th District Court in Bexar County, where judges rotate and none has yet been assigned to try the case. Ms. Ramirez was implanted on Sept. 17, 2010 with a Johnson & Johnson subsidiary Ethicon product, TVT-O mesh, the same type which was implanted in Ms. Linda Batiste and found defective by a Dallas Jury earlier this year. Ms. Ramirez, a mother of three, was 28 when the mesh was implanted in her to treat stress urinary incontinence. After the surgery, she complained of intense pain and also continuing stress urinary incontinence. She then had surgeons attempt to remove the mesh three months later, on Dec. 22, 2010. The complaint charges that portions of the mesh remain inside of her, despite explant surgery, and the intractable mesh contributes to her ongoing pain, while she also still suffers from stress urinary incontinence.
Ramirez vs. Johnson & Johnson (also Reyes, et al.) is scheduled to begin Jan. 12, 2015 in San Antonio, in 438th District Court in Bexar County, where judges rotate and none has yet been assigned to try the case. Ms. Ramirez was implanted on Sept. 17, 2010 with a Johnson & Johnson subsidiary Ethicon product, TVT-O mesh, the same type which was implanted in Ms. Linda Batiste and found defective by a Dallas Jury earlier this year. Ms. Ramirez, a mother of three, was 28 when the mesh was implanted in her to treat stress urinary incontinence. After the surgery, she complained of intense pain and also continuing stress urinary incontinence. She then had surgeons attempt to remove the mesh three months later, on Dec. 22, 2010. The complaint charges that portions of the mesh remain inside of her, despite explant surgery, and the intractable mesh contributes to her ongoing pain, while she also still suffers from stress urinary incontinence.
Ms. Ramirez is represented by the same law firms that won the $1.2 Million verdict in Dallas in the last TVT-O case to be tried against Johnson & Johnson and Ethicon – Matthews & Associates and Freese & Goss.
Perry vs. Johnson & Johnson – 1/12/15
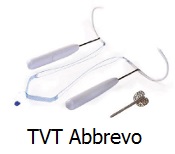 Perry vs. Johnson & Johnson (also Luu, et al.) is scheduled to be tried in Kern County Superior Court in the State of California beginning Jan. 12, 2015. Ms. Perry was 47 on March 3, 2011 when she was implanted with a Johnson & Johnson Ethicon product, the TVT-Abbrevo, to treat her stress urinary incontinence. She then had surgery attempting to remove the mesh on Jan. 17, 2012.
Perry vs. Johnson & Johnson (also Luu, et al.) is scheduled to be tried in Kern County Superior Court in the State of California beginning Jan. 12, 2015. Ms. Perry was 47 on March 3, 2011 when she was implanted with a Johnson & Johnson Ethicon product, the TVT-Abbrevo, to treat her stress urinary incontinence. She then had surgery attempting to remove the mesh on Jan. 17, 2012.
Ms. Perry experienced mesh erosion and exposure and had some of the mesh excised. She also complains of pain, urinary problems, and dyspareunia. Her case against Ethicon will be the first trial involving the Johnson & Johnson TVT-Abbrevo product nationwide. Ms. Perry will be represented by Matthews & Associates and Freese & Goss.
Rabiola vs. Johnson & Johnson – 2/23/15
 Rabiola vs. Johnson & Johnson (also Antonini, et al.) is scheduled to begin Feb. 23, 2015 in the 53rd Judicial District Court of Travis County in Austin, Texas. Ms. Rabiola had surgery for both pelvic organ prolapse (POP) and for stress urinary incontinence (SUI). She was implanted at age 63 with a Johnson & Johnson Ethicon product, TVT-S, for SUI on May 24, 2010; and then she was implanted with Johnson & Johnson product Prosima (pictured, recalled in 2012) for POP on Nov. 2, 2011.
Rabiola vs. Johnson & Johnson (also Antonini, et al.) is scheduled to begin Feb. 23, 2015 in the 53rd Judicial District Court of Travis County in Austin, Texas. Ms. Rabiola had surgery for both pelvic organ prolapse (POP) and for stress urinary incontinence (SUI). She was implanted at age 63 with a Johnson & Johnson Ethicon product, TVT-S, for SUI on May 24, 2010; and then she was implanted with Johnson & Johnson product Prosima (pictured, recalled in 2012) for POP on Nov. 2, 2011.
Ms. Rabiola’s health problems following her implants include mesh erosion, recurrent bladder issues, mesh exposure/extrusion. She will be represented in California court by Matthews & Associates and Freese & Goss.
Related
- J&J Mesh Approved by FDA Based on Recalled Device
- Medical Device Oversight – 510(k)
- Boston Scientific Mesh on Trial in Dallas Again

by Matthews & Associates




