Mectronic’s Infuse might be effective in the one surgical use for which it was approved by the FDA – in the lumbar spine for single-level, frontal approach fusion surgeries – but its off-label use has caused serious, sometimes life-threatening problems.
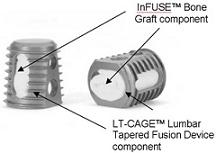 Infuse is the trade name for bone morphogenetic protein – or BMP-2 – which is packed inside a thimble-sized (LT) cage implanted in a patient to encourage bone growth. The problem is that Medtronic aggressively moved to market Infuse for many off-label uses without first doing the proper research to ensure that Infuse would work for those uses.
Infuse is the trade name for bone morphogenetic protein – or BMP-2 – which is packed inside a thimble-sized (LT) cage implanted in a patient to encourage bone growth. The problem is that Medtronic aggressively moved to market Infuse for many off-label uses without first doing the proper research to ensure that Infuse would work for those uses.
Medtronic’s Infuse hit national news in October 2012 when a U.S. Senate investigation referenced Medtronic’s paying 13 doctors $210 million to manipulate data in order to profit from expanded applications of the product despite limited FDA approval.
The Senate query was partly prompted by the release last year of a special issue of The Spine Journal, in which a group of spine specialists publicly repudiated studies performed by Medtronic-financed researchers. The spine specialists said Medtronic had understated serious Infuse-related adverse events, including male sterility. The Medtronic-financed researchers involved responded that their reports were accurate.
The Senate inquiry also found internal Medtronic documents indicating the company had inserted language into reports claiming that Infuse was superior to a bone graft because it eliminated the pain associated with harvesting bone from a patient’s pelvis. Medtronic said that there was a medical debate about the complications of bone graft use and that any suggestions that it used that issue for marketing purposes was wrong.
“Patients everywhere will be better served by a more open, honest system without this kind of collusion,” the chairman of the Senate Finance Committee, Max Baucus, Democrat of Montana, said in a statement.
In addition, “Medtronic’s FDA Data on Infuse Contradict Published Studies” was the title of a June 2011 Medscape article (see the full story), which also showed a shocking discrepancy between Medronic’s data and that which was reported in actual studies. Medtronic’s massaging of published study data should be very troubling for anyone concerned with truth in reporting and the public’s health and general welfare.
Off-label use of Infuse in cervical spine procedures, such as lateral or posterior approach spinal surgeries, has been linked to serious and even life-threatening “side effects.”
“Side effects” of Infuse off-label include:
• death – some patients have reportedly died after suffering acute neck swelling following off-label use
• ectopic or uncontrolled bone growth near the surgery site
• difficulty breathing, talking, swallowing
• radiating pain in the legs or arms
• male sterility
• retrograde ejaculation and/or other uro-genital injuries
• nerve damage causing severe or chronic pain
• cancer – potentially, in many different forms
Matthews & Associates has filed Infuse cases against Medtronic in several states so far. Contact an experienced Infuse attorney at the law firm if you or someone you love is suffering from off-label use of Infuse.
FacebookTwitter
by Matthews & Associates




