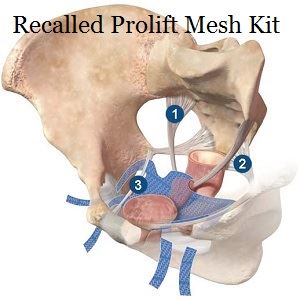
 (July 7, 2018) The mainstream media has finally recognized that a huge number of women are suing mesh manufacturers over their plastic products. A CBS news story reported last month that nearly 50,000 women are suing one mesh maker alone over gynecological mesh. The polypropylene (plastic) mesh is often used to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI), which affect millions of women. At least 100,000 women across the country are suing over gynecological mesh. Many have filed their cases in the multidistrict litigation court in West Virginia. Several other pelvic mesh lawsuits have been filed in various state courts throughout the country.
(July 7, 2018) The mainstream media has finally recognized that a huge number of women are suing mesh manufacturers over their plastic products. A CBS news story reported last month that nearly 50,000 women are suing one mesh maker alone over gynecological mesh. The polypropylene (plastic) mesh is often used to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI), which affect millions of women. At least 100,000 women across the country are suing over gynecological mesh. Many have filed their cases in the multidistrict litigation court in West Virginia. Several other pelvic mesh lawsuits have been filed in various state courts throughout the country.
Related: Transvaginal Mesh Lawsuit | Pelvic Mesh
Gynecological Mesh Makers
The CBS news story mentioned only Boston Scientific as a defendant in transvaginal mesh (TVM) cases, though several other gynecological mesh makers also face lawsuits. Other defendants include Johnson & Johnson (Ethicon), American Medical Systems (AMS), C.R. Bard, Coloplast, and Cook Medical.
Sixty Minutes reported that Boston Scientific faces some 48,000 lawsuits in what has generated the largest multi-district litigation since asbestos. The suits claim mesh can inflict life-changing pain and injury. Suits against Boston Scientific also claim the company used a mesh product never meant to be put inside the human body.
Related: Johnson & Johnson Mesh Verdicts Mount
More than two million American women have received gynecological mesh implants. All the mesh makers claim their products are safe.
CBS News
CBS News reported the story of one woman implanted with a Boston Scientific gynecological mesh for stress urinary incontinence and also to lift organs that shifted after pregnancy. Gwyn Madsen had a Boston Scientific implant in 2012.
Gwyn Madsen told CBS that the mesh “felt like a cheese grater inside of me.”
Ms. Madsen said she suffered pain which left her barely able to sit or play with her children. She said, “It felt like the material was pulling on the muscles and I’d get shooting pains. You almost felt like there was something inside of you that was like sandpaper back and forth, every time you’d walk.”
Boston Scientific, which lost a $73 million verdict over one of its TVM products following a 2016 trial in Texas, has fought allegations like Ms. Madsen’s for years. The company told CBS: “Nearly one million women have been successfully treated. (We) have extensively tested the [plastic] resin to confirm its composition, safety and performance.”
100,000 Women suing over Gynecological Mesh
The American Urogynecological Society – which has embraced the plastic mesh because it is easier to apply than the gold standard suture method for repairing POP or SUI – has also claimed plastic mesh is “safe and effective.” Many doctors, however, disagree.
Dr. Michael Margolis testified for the woman who won a $73 million verdict in the Texas case. He told CBS that the women’s mesh causes a chronic inflammatory reaction. He has removed more than 350 mesh implants.
“The slings I’ve removed are substantially altered in their architecture,” Dr. Margolis told CBS. “They are shrunk by at least 50% in width. They are encased in scar tissue. The pores [in the mesh] shrunk substantially.”
Dr. Margolis showed CBS a mesh he removed which had been implanted. It had shrunk substantially, he said, had folded, contracted, embedded in scar tissue, and was choking off the woman’s urethra. He said it was half the size it had been upon implant.
Dr. Margolis told Scott Pelley the implant is “not supposed to change.”
Missing from the CBS story and interviews was the fact that Boston Scientific, as well as Johnson & Johnson (Ethicon, American Medical Systems (AMS), and Bard have all lost multi-million-dollar verdicts to women implanted with the companies’ transvaginal mesh products.
FDA: Serious Adverse Events “not rare”
The story did include the fact that the FDA has issued a damning report regarding TVM mesh. In 2011, the FDA said that it found mesh used to support organs after pregnancy had resulted in nearly 4,000 “reports of injury, death, and malfunction” and complications including “pain, infection, urinary problems, bleeding and organ perforation.” The FDA wrote that, “Serious adverse events are not rare.”
Indeed. At least 100,000 women would apparently agree with that statement.
Related
- Transvaginal Mesh Lawsuit | Pelvic Mesh Lawsuit
- $73 Million TVM Verdict
- Johnson & Johnson illegally destroyed Mesh Evicdnce
- Transvaginal Mesh Catastrophe
- ProLift Mesh Recalled

by Matthews & Associates




